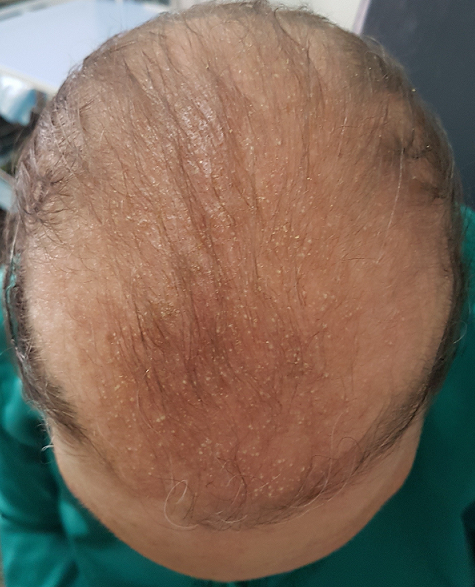
Pustular dermatosis of the scalp due to topical minoxidil 5%
Ouiam El Anzi, Badreddine Hassam
PAMJ. 2018; 30:83. Published 29 May 2018 | doi:10.11604/pamj.2018.30.83.15384
Corresponding author
Ouiam El Anzi, Service de Dermatologie et Vénérologie, Centre Hospitalier Universitaire Ibn Sina, Faculté de Médecine et de Pharmacie, Université Mohammed V, Rabat, Maroc (elanzi.ouiam@gmail.com)
This image
| Articles published in PAMJ are Open Access and distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0). |  |
eISSN: 1937-8688
The Pan African Medical Journal (ISSN: 1937-8688) is a subsidiary of the Pan African Medical Journal. The contents of this journal is intended exclusively for professionals in the medical, paramedical and public health and other health sectors.
Currently tracked by: DOAJ, AIM, Google Scholar, AJOL, EBSCO, Scopus, Embase, IC, HINARI, Global Health, PubMed Central, PubMed/Medline, ESCI
Physical address: "Kenya: 3rd Floor, Park Suite Building, Parkland Road, Nairobi. PoBox 38583-00100, tel: +254 (0)20-520-4356 | Cameroon: Immeuble TechnoPark Essos, Yaounde, PoBox: 10020 Yaounde, tel: +237 (0)24-309-5880"





